
At present, breast cancer still ranks first among female tumors in China, seriously endangering the life and health of Chinese women, so it is also known as the "first killer" of women's health. Although the incidence rate of breast cancer is high, the prognosis of most patients is relatively optimistic, except for the most difficult subtype - triple negative breast cancer.
Triple negative breast cancer (TNBC) is characterized by the loss of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2), accounting for 15-20% of all invasive breast cancer, and is associated with early recurrence and high risk of death. Adjuvant anthracycline/taxane chemotherapy is the standard treatment for early triple negative breast cancer, but about 20-40% of patients will have disease recurrence. Therefore, there is an urgent need for more effective strategies to optimize the adjuvant treatment of triple negative breast cancer.
On October 23, 2024, a team led by Professor Shao Zhimin, Professor Wang Zhonghua, and Professor Jiang Yizhou from Fudan University Affiliated Cancer Hospital published an article titled "Intensive chemotherapy versus standard chemotherapy among patients with high risk" in the top medical journal BMJ (IF=93.6), A research paper titled 'Operable, Triple Negative Breast Cancer Based on Integrated mRNA-LncRNA Signature (BCTOP-T-A01): Randomized, Multicentric, Phase 3 Trial'.
This study explored the feasibility of customized adjuvant chemotherapy with multiple gene markers in operable triple negative breast cancer patients,
The research results confirmed that: based on the "multi gene model" independently developed by the tumor hospital, targeted at triple negative breast cancer, the "anthracycline taxus" sequential "gemcitabine" combined with "carboplatin" precision treatment program has significantly improved the survival rate of high-risk patients by more than 10%, changing the traditional triple negative breast cancer adjuvant chemotherapy "one thousand people per party" situation.

In 2015, Professor Shao Zhimin and Professor Jiang Yizhou from the Cancer Hospital affiliated to Fudan University independently developed and constructed a three negative breast cancer prognosis prediction model (referred to as "multi gene model") consisting of five RNAs based on the full transcriptome expression profile.
This is the first three negative prognosis prediction model for breast cancer in the world, and related research results were published in Cancer Research.
In order to further verify the prognostic effect of the "multi gene model" and explore its clinical application value, Professor Shao Zhimin led a team to conduct a clinical study called "BCTOP-T-A01". The study lasted for 7 years, from January 3, 2016 to July 17, 2023, a total of 504 early (M0) triple negative breast cancer patients aged 18-70 years were included. These patients all received unilateral invasive breast cancer surgery in Fudan University Cancer Hospital, and have not received new adjuvant treatment in the past. The research subjects include patients with positive lymph nodes and those with negative lymph nodes but tumors larger than 1 centimeter in size.

The triple negative breast cancer patients were divided into high-risk and low-risk recurrence risk groups through the prediction model, with a median follow-up time of 45 months.
High risk patients were randomly (1:1) divided into two groups:
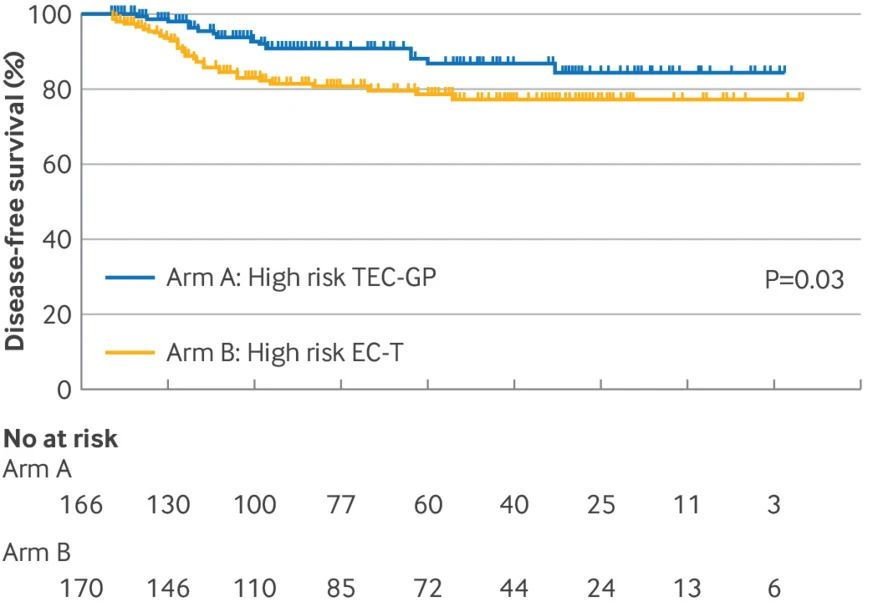
Group A: Intensive treatment, including 4 cycles of docetaxel, epirubicin, and cyclophosphamide, followed by 4 cycles of gemcitabine and cisplatin (n=166).
Group B: Standard treatment, 4 cycles of epirubicin and cyclophosphamide, followed by 4 cycles of docetaxel (n=170).
Low risk patients received the same adjuvant chemotherapy as group B (group C; n=168).
Primary endpoint: Disease free survival rate between Group A and Group B.
Secondary endpoints: Disease free survival, recurrence free survival, overall survival, and safety between Group C and Group B.
The 3-year survival rate of high-risk patients has increased by 10.3%
The research results show that the disease-free survival rate is 90.9% in group A and 80.6% in group B.
The recurrence free survival rate was 92.6% in Group A and 83.2% in Group B.
Overall survival rate: The 3-year overall survival rate for Group A was 98.2%, while for Group B it was 91.3%.
Low risk group (Group C):. The 3-year disease-free survival rate (90.1% compared to 80.6%), recurrence free survival rate (94.5% compared to 83.2%), and overall survival rate (100% compared to 91.3%) of the low-risk group were all better than those of high-risk patients receiving the same standard chemotherapy regimen (Group B).

In addition, regarding the exploration of security:
8 cases (5%), 5 cases (3%), and 4 cases (2%) of patients in groups A, B, and C stopped the study treatment due to toxicity, respectively. In randomized patients receiving study treatment, the incidence of treatment-related adverse events of grade ≥ 3 in group A was significantly higher than that in group B, but there was no significant difference in the incidence of treatment-related serious adverse events or treatment-related adverse events leading to discontinuation between group A and group B. No treatment-related deaths occurred in the study.
In summary, the precision chemotherapy regimen guided by the multi gene model significantly improved the survival rate of high-risk patients by over 10%, while also increasing the survival rate of low-risk patients by nearly 10%, strongly demonstrating the application value of this model.
"While significantly improving the prognosis of high-risk triple negative breast cancer patients, the safety of this treatment scheme is controllable, and patients have no serious adverse reactions, reflecting more advantages of this scheme." Professor Shao Zhimin, director of breast surgery and chief of major surgery of Fudan University Cancer Hospital, said.

Professor Jiang Yizhou said that "BCTOP-T-A01" was the first clinical study carried out by the breast cancer precision treatment collaboration group (BCTOP) led by Professor Shao Zhimin. The mode of multi center cooperation and major achievements fully prove the purpose of BCTOP - "based on the concept of precision medicine, carry out multi center clinical research, promote transformation research platform, develop new drugs and technologies, and ultimately improve the diagnosis and treatment level and international status of breast cancer in China."
It is reported that the tumor hospital is preparing to carry out step-down treatment for low-risk triple negative breast cancer patients predicted by the model. It is believed that in the future, "BCTOP" will bring more high-quality "China Program" to the world and benefit more breast cancer patients.