
Cancer metastasis refers to the process in which cancer cells detach from
the primary tumor site, spread through the blood or lymphatic system to other
parts of the body, and form new tumors in these new locations. This process is
the main cause of most cancer-related deaths. The transfer process usually
includes the following steps: local invasion, vascular infiltration (entering
the bloodstream), survival in circulation, extravasation (leaving the
bloodstream and entering the target tissue), and growth and proliferation in a
new location.
Although certain lipid related changes are known to be crucial for the
extravasation of cancer cells, there is still much unknown about which specific
lipid components support the survival and growth of cancer cells in new
environments.
On November 25, 2024, the team led by Zou Yilong and Wang Xi from Xihu
University collaborated with Dr. Cao Jian from Nanjing Maternal and Child Health
Hospital to publish a research paper titled "ACSL4 and polyunsaturated lipids
support metastatic extravasation and colonization" in the top international
academic journal Cell.
Dr. Wang Yuqi, Assistant Researcher at Xihu Laboratory, PhD student Hu
Xize, and Dr. Cao Jian from Nanjing Maternal and Child Health Hospital/Nanjing
Medical University Affiliated Obstetrics and Gynecology Hospital are co first
authors of the paper.
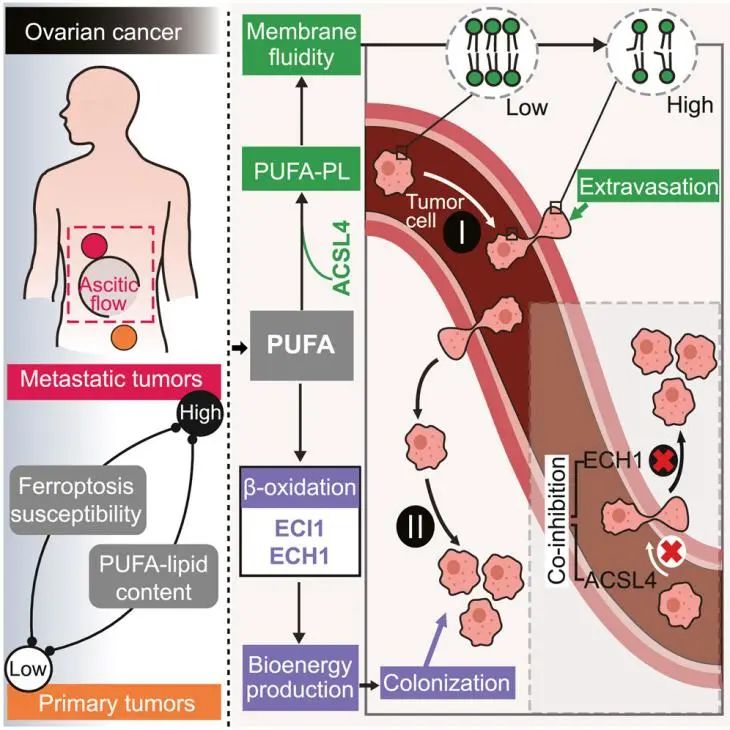
This study revealed the association between metastatic potential and iron
death sensitivity in various cancers. Cancer cells isolated from ovarian cancer
patients with metastatic foci showed higher iron death sensitivity and
polyunsaturated fatty acid (PUFA) - lipid content compared to cells from primary
tumors. Furthermore, it was found that ACSL4 promotes blood transmission and
metastasis, providing a new target for therapeutic development.

Research Highlights
Pan cancer phenotype data mining reveals correlation between metastasis
iron apoptosis sensitivity
In vivo CRISPR screening with a focus on metabolism reveals the role of
ACSL4 in promoting metastasis
ACSL4 promotes metastatic extravasation by enhancing membrane fluidity and
invasion
research contents
Previous studies have found that cancer cells that reach distant organs may
have evolved an optimal lipid metabolism profile that is favorable for their
survival during circulation and extravasation. These cancer cells meet their
high energy demands in low nutrient environments by activating mitochondrial
biogenesis and oxidative phosphorylation. β - oxidation plays a crucial role in
this process.
β - oxidation is the cleavage of fatty acids between alpha and beta carbon
atoms under the action of a series of enzymes, producing acetyl CoA and fatty
acyl CoA with two fewer carbon atoms than before. Beta oxidation plays a crucial
role in supporting the growth of cancer cells in vivo by providing fuel for
mitochondria with high flux of CoA.
The research team found through analysis of a pan cancer phenotype database
that certain cancer types with high metastatic potential, such as ovarian
cancer, liver cancer, and head and neck cancer, exhibit higher sensitivity to
iron induced apoptosis. Further research has confirmed the susceptibility to
ferroptosis and increased content of polyunsaturated fatty acyl (PUFA) - lipids
in metastatic tumor cells.
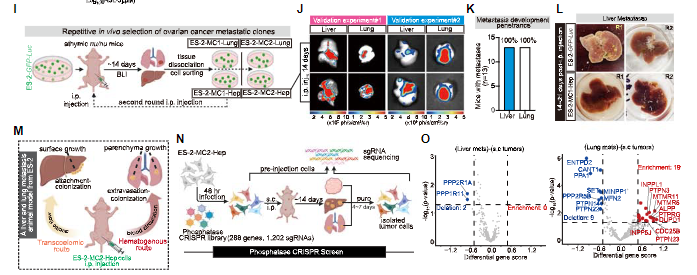
Subsequently, through in vivo CRISPR screening, several metabolic genes
promoting metastasis were identified in the established mouse model of ovarian
cancer distant metastasis, including NMNAT 1 involved in NAD production and ACSL
4 involved in PUFA lipid biosynthesis. Among them, ACSL 4 can specifically
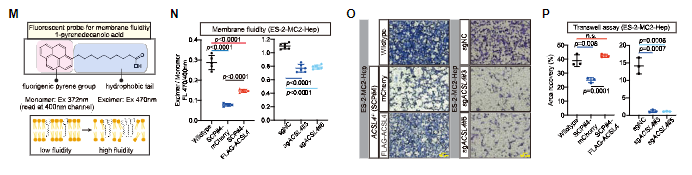
mediate metastatic extravasation during hematogenous dissemination by enhancing
membrane fluidity and promoting migration.
At the end of the ACSL 4 depletion experiment, ARA (a rich PUFA)
pretreatment increased the PUFA lipid content of ES-2-GFP Luc parental cells and
increased the tumor metastasis and extravasation rate in mice after intravenous
implantation.
Researchers have found that ACSL4 enhances the iron death process by
promoting the esterification of polyunsaturated fatty acids (PUFAs) to acyl CoA.
In addition, metastatic cancer cells with high PUFA lipid content rely on β -
oxidation of unsaturated fatty acids (UFA) to survive in the tumor
microenvironment.
MDH1, ABHD6, ECI1, and ECH1 are key rate limiting enzymes involved in UFA β
- oxidation, playing an important role in preparing UFA for metabolic
breakdown.
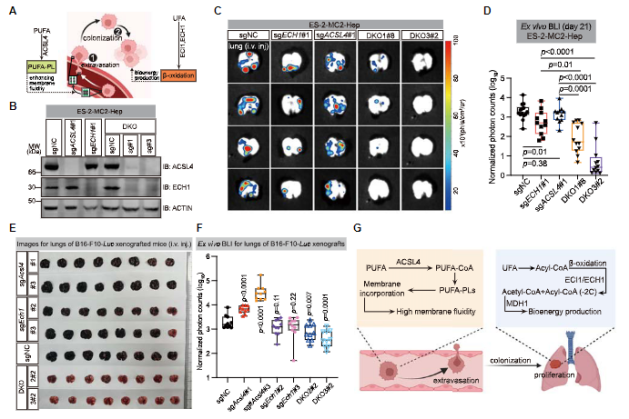
Researchers have found that blocking UFA esterification (via ACSL4) and
beta oxidation (via ECH1) can significantly reduce the growth and colonization
of metastatic tumors. And this combined inhibition strategy is not only
applicable to specific types of cancer, such as ovarian cancer, but also to
other types of cancer, such as melanoma.
In summary, these findings suggest the possibility of inhibiting cancer
metastasis by targeting lipid metabolism pathways. In addition, the study also
demonstrated the broad applicability of this strategy in different cancer types
and immune active environments, laying the foundation for further clinical
applications.
This article is also a product of the strong collaboration between
researchers and clinical doctors. Clinical doctors have a natural advantage in
conducting scientific research. They directly face patients, understand their
real clinical needs, and are able to transform clinical problems into scientific
research topics. By collaborating with basic research scholars for in-depth
basic research, research results can be quickly applied to clinical practice,
accelerating the transformation of results and bringing more effective treatment
plans to patients.
With the continuous emergence of research-oriented doctors, their status in
the scientific research community will continue to rise. In the future, we have
reason to believe that this closely integrated model of clinical and scientific
research will bring more breakthroughs and innovations to the medical field.