
In cancer and chronic viral infections, exhausted T cells (TEX) undergo a
series of metabolic and epigenetic remodeling, weakening their immune protective
abilities. However, it has not been clear how nutritional metabolism
specifically affects epigenetic modifications that control TEX
differentiation.
On December 13, 2024, Ma Shixin, a countrymen scholar from the Susan M.
Kaech team of the Salk Institute of Biology, as the first author, published a
research paper entitled "Nutrient driven histone code decisions evolved CD8+T
cell factors" on Science, revealing the key influence of nutrition metabolism on
the fate of depleted CD8+T cells (TEX) and the underlying mechanism.
Research has shown that in cancer and chronic viral infections, TEX cells
shift from acetate metabolism to citrate metabolism pathway by downregulating
acetyl CoA synthase 2 (ACSS2) while maintaining ATP citrate lyase (ACLY)
activity. This metabolic conversion increases citrate dependent histone
acetylation, particularly on TEX characteristic genes, while reducing acetate
dependent histone acetylation on effector and memory T cell genes.
Overexpression of ACSS2 or inhibition of ACLY in the nucleus can prevent TEX
differentiation and enhance tumor specific T cell response.
This study reveals a nutrition driven histone code that determines the fate
of CD8+T cells, providing important theoretical basis and practical guidance for
novel T cell therapies based on metabolism and epigenetics.

In addition to transcription and epigenetic reprogramming, metabolic
reprogramming is also an important feature of TEX cells. Effect T cells (TEFF)
support their growth, proliferation, and effector function through efficient
glucose and amino acid metabolism, while TEX cells undergo a gradual shift in
their nutritional metabolism pattern, characterized by decreased mitochondrial
function, increased dependence on glycolysis, and increased uptake of oxidized
lipids, which impair their functional capacity.
Metabolic intermediates, such as acetyl CoA, act as substrates for histone
acetylation and participate in modifying the cell's epigenome. In mammalian
cells, acetyl CoA is mainly synthesized from acetate and citrate by acetyl CoA
synthase 2 (ACSS2) and ATP citrate lyase (ACLY). However, it is currently
unclear whether the acetyl CoA generated by these enzymes (such as ACSS2 and
ACLY) plays an important role in determining the unique epigenetic features and
differentiation patterns of CD8+T cells in tumors and chronic infections.
To explore the role of nutritional metabolism in epigenetic modifications
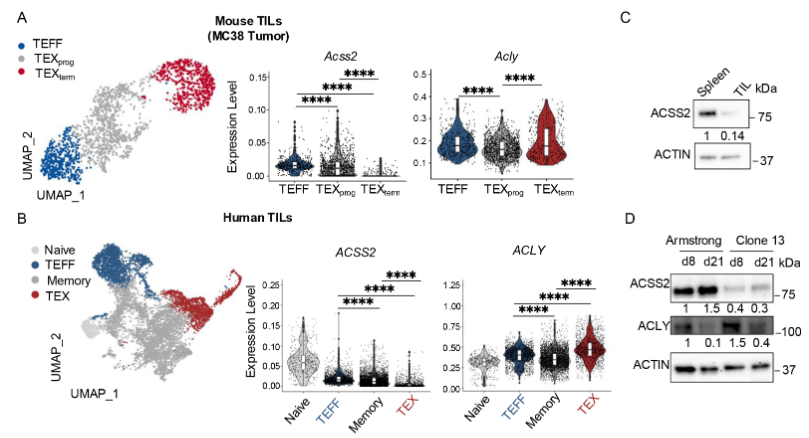
during CD8+T cell differentiation. The researchers first validated the
differential expression of ACSS2 and ACLY in TEFF and TEX cells through
transcriptome analysis and Western blot. The results showed that TEX cells
downregulated ACSS2 expression while maintaining ACLY expression during tumor
and chronic infection. Does it mean that these two modes have opposite
effects?
Subsequently, the research team used gene knockout methods to investigate
the roles of ACSS2 and ACLY in the formation of TEX cells during tumorigenesis
and chronic LCMV infection. It was found that in the case of T cell failure, the
absence of Acss2 weakens the activity of CD8+T cells, while the absence of Acly
enhances their function.
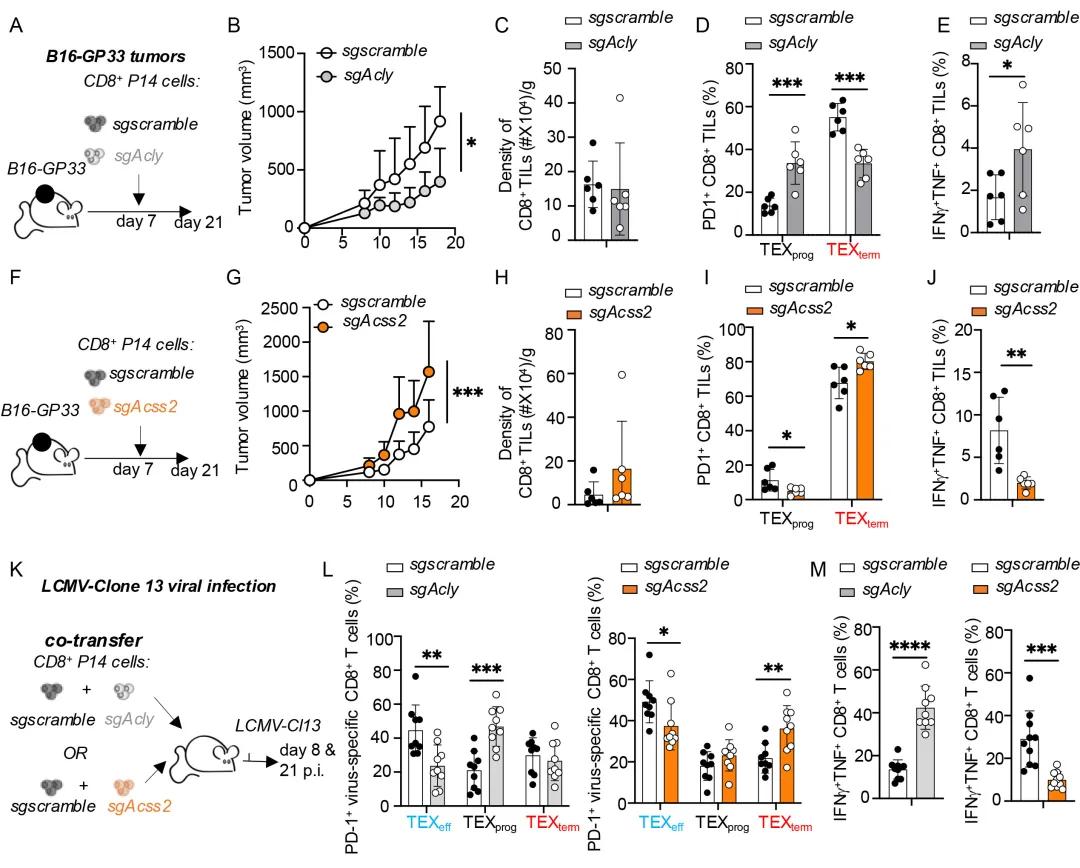
This raises a new scientific question: Does the acetyl CoA generated by
ACLY and ACSS2 determine the formation of TEX?
The research team tracked the pathway of acetic acid and glucose metabolism
to produce acetyl CoA through isotope tracing experiments, and combined histone
acetylation analysis and CUT&Tag sequencing technology to explore in depth
how ACSS2 and ACLY regulate the histone acetylation levels of TEXProg and
TEXterm related genes through p300 and KAT2A histone acetyltransferases,
respectively. Research has found that ACSS2 mainly promotes histone acetylation
of functional effector T cell (TEFF) genes through p300 under acute stimulation,
while ACLY enhances acetylation of exhausted T cell (TEXterm) genes through
KAT2A under chronic stimulation.
Further research utilized ACSS2 overexpression vectors labeled with nuclear
localization signals and ACLY inhibitor BMS-303141 to evaluate the potential of
these interventions in restoring T cell function and enhancing anti-tumor immune
response. The results showed that overexpression of ACSS2 or inhibition of ACLY
not only prevented the differentiation of TEX cells, but also significantly
enhanced the anti-tumor activity of T cells.

In summary, this study reveals for the first time that although the
intermediate products of nutritional metabolism are the same, different types of
nutrients play a crucial role in regulating cell fate and function, providing a
new perspective for studying cellular nutritional metabolism. By delving into
the roles of ACSS2 and ACLY in T cell exhaustion (TEX), this work not only
deepens our understanding of the TEX mechanism, but also provides important
practical guidance for improving existing immunotherapy strategies. Its research
results are expected to promote the development of personalized medicine,
especially in the treatment of cancer and chronic infections, demonstrating
broad application prospects.