
Efficient differentiation scheme for venous endothelial cells (iVEC)
With the support of National Natural Science Foundation projects (approval
numbers: 82370514, 82472171) and other grants, the teams of Researcher Wang Kai,
Professor Kong Wei, Researcher Wang Qian, and Researcher Xie Zhengwei from
Peking University have made progress in inducing pluripotent stem cells for
vascular malformation modeling and drug discovery research. The research
results, titled "Generation of iPSC derived human venous endothelial cells for
modeling of vascular malformations and drug discovery", were published online on
November 22, 2024 in the journal Cell Stem Cell. Paper link:
https://www.sciencedirect.com/science/article/pii/S1934590924003771?via%3Dihub
.
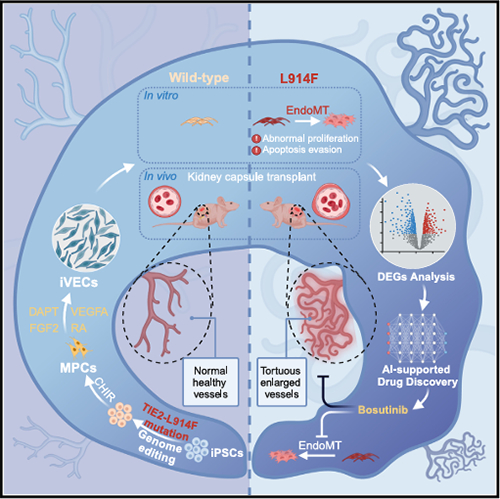
Venous malformations (VMs) are common vascular abnormalities, mainly caused
by non genetic somatic mutations in venous endothelial cells (VECs). At present,
there is a lack of mature models for VMs disease, which seriously hinders the
discovery of drugs for treating VMs.
The research team has developed an efficient method for inducing induced
venous endothelial cells (iVECs) in humans. Using gene editing technology, the
L914F mutation was introduced into the TIE2 locus of induced pluripotent stem
cells (iPSCs), and it was demonstrated that the mutated iVECs formed dilated
blood vessels after transplantation into mice, thereby reproducing the
phenotypic characteristics of VMs, including disrupted cytoskeleton, enhanced
proliferation ability, and reduced luminal ability. In addition, the team
conducted drug screening by combining deep neural networks with high-throughput
digital RNA with perturbation of genes sequencing (DRUG seq) technology, and
discovered a potential therapeutic drug for VMs, Bosutinib. By weakening the
endothelial mesenchymal transition of mutant iVECs, inhibiting cell
proliferation, and effectively improving the disease phenotype of VMs. The
research team utilized genome editing and stem cell technology to construct a
VMs disease model, laying the foundation for further development of novel drugs
for treating VMs (Figure).