
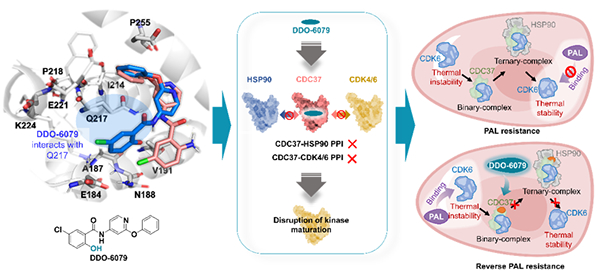
Figure DDO-6079 conformational binding to CDC37 and selective inhibition of
kinase protein maturation
Under the support of National Natural Science Foundation of China projects
(approval numbers: 82173741, 81930100, 82304309, 81925034), researchers Wang Lei
and Professor You Qidong from China Pharmaceutical University have collaborated
with Professor Zhang Jian's team from Shanghai Jiao Tong University to make
progress in the design of targeted molecular chaperone system drugs. The
research result titled "CDC37 allosteric inhibitor can disrupt chaperone complex
to block CDK4/6 maturation" was published online on November 24, 2024 in the
journal Angewandte Chemie International Edition. Paper link:
https://onlinelibrary.wiley.com/doi/10.1002/anie.202413618 .
The molecular chaperone system is responsible for regulating the mature
folding and post-translational modifications of numerous substrate proteins,
which is crucial for maintaining normal physiological functions in living
organisms. The co chaperone protein of the heat shock protein 90 (HSP90)
chaperone system, cell division cycle 37 (CDC37) protein, is responsible for
recruiting kinases to be folded and matured in the HSP90 chaperone system, which
then interacts with the HSP90 protein to deliver the recruited client kinases to
the HSP90 protein. CDC37 binds to both HSP90 and kinase, and is overexpressed in
tumor cells, making it a potential drug target. However, there are complex
protein interactions between CDC37, HSP90, and kinase, making it difficult for
small molecules to simultaneously intervene in the protein interactions of
HSP90-CDC37 and CDC37 kinase. Currently, there are no specific CDC37 small
molecule inhibitors reported.
This study first constructed a screening system for in vitro affinity and
protein interaction inhibition. Through compound library screening, promising
molecules with CDC37 binding activity were discovered. Subsequently, structure
based drug design was carried out to modify the structure of the precursor
molecules, resulting in the acquisition of a small molecule DDO-6079 with better
binding activity. Mechanism studies have found that this molecule binds to a
novel conformational site of CDC37, and through conformational regulation, it
can simultaneously achieve synergistic inhibition of HSP90-CDC37 and
CDC37-CDK4/6 chaperone complex. DDO-6079 can selectively inhibit the maturation
of various oncogenic kinases while avoiding the heat shock toxic side effects of
classical HSP90 inhibitors. In addition, DDO-6079 can also reduce the thermal
stability of CDK6, reverse the resistance of colorectal cancer cells to
palbociclib (CDK4/6 inhibitor), and show strong anti colorectal cancer activity
in animals (Figure).
This study not only reported the first CDC37 small molecule allosteric
inhibitor DDO-6079, but also discovered a novel biological function of targeting
CDC37 to reduce the thermal stability of CDK6, achieving the reversal of
existing CDK4/6 drug resistance, providing research strategies and methods for
precise regulation of molecular chaperone systems, and potential targets for
drug design targeting molecular chaperone systems.