
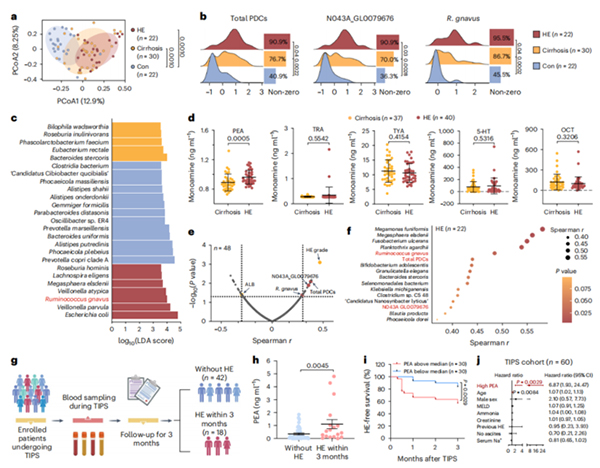
The serum PEA level in patients with liver cirrhosis is correlated with the abundance of fecal PDC coding genes, and patients with high serum PEA levels before TIPS surgery have a higher risk of developing HE after TIPS surgery
Under the support of National Natural Science Foundation of China projects
(approval numbers: 81925026, 82272387, 31900101, 82130068, 82372305), the teams
of Professor Zhou Hongwei and Professor Chen Jinjun from Southern Medical
University, together with Professor Gao Jie from Guangzhou Medical University
and Professor Qi Xiaolong from Southeast University, have made progress in the
study of the mechanism of hepatic encephalopathy (HE) driven by intestinal
microbiota imbalance. The related research results, titled "The gut brain axis
underlying hepatic encephalopathy in liver cirrhosis", were published online on
January 8, 2025 in the journal Nature Medicine. The link to the paper is:
https://www.nature.com/articles/s41591-024-03405-9 .
Hepatic encephalopathy is a common and serious complication in patients
with cirrhosis, with a complex pathogenesis. According to traditional theory,
the pathogenesis of HE is mainly attributed to "ammonia poisoning", which refers
to the inability of the liver to effectively remove ammonia from the blood,
resulting in the accumulation of ammonia in the brain and causing nerve damage.
However, blood ammonia levels are not fully correlated with the occurrence and
severity of HE, indicating the existence of other unexplained causes of hepatic
encephalopathy in cirrhosis.
The research team first constructed a gene set that can evaluate the neural
activity metabolites derived from gut microbiota, known as the "gut brain
module". By analyzing the gut brain module in the gut microbiota metagenomic
data of patients with cirrhosis, it was found that the monoamine synthesis
module was significantly enriched. Further enzyme activity screening experiments
confirmed that phenylalanine decarboxylase (PDC) from Ruminococcus gnavus is a
key rate limiting enzyme in the microbial monoamine synthesis module. Animal
experiments have confirmed that the colonization of Vibrio rumenalis in
cirrhotic mice can lead to the accumulation of phenylethylamine (PEA) in their
brains, and exhibit typical hepatic encephalopathy symptoms such as memory
impairment and flapping tremors. Using fecal microbiota transplantation
experiments, the research team found that liver cirrhosis mice colonized with
fecal microbiota from HE patients exhibited significant memory impairment, and
the use of PDC inhibitors or PEA neutralizing antibodies could effectively block
the neurotoxic effects of fecal microbiota from HE patients. Finally, the team
found that the abundance of active rumen bacteria and PDC genes in the feces of
HE patients was significantly higher than that of non HE cirrhosis patients, and
positively correlated with serum PEA levels; In the cohort of liver cirrhosis
patients undergoing intrahepatic portal vein shunting, patients with high
preoperative PEA levels had a 7-fold higher risk of developing HE compared to
patients with low PEA levels (Figure).
This study reveals a new mechanism by which gut active rumen bacteria drive
hepatic encephalopathy through PDC and its product PEA, providing a potential
new target for the precise diagnosis and treatment of HE.