
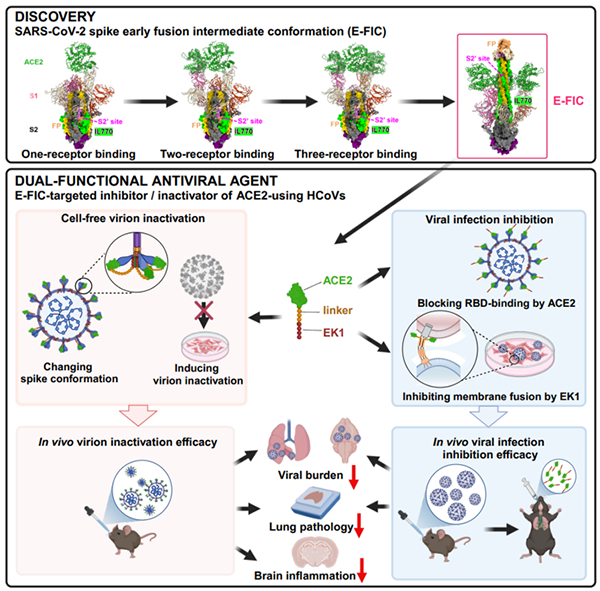
Figure Schematic Diagram of Discovery of Early Fusion Intermediate Target of COVID-19 Spike Protein and Design and Verification of Antiviral Drugs
With the support of National Natural Science Foundation of China projects (approval numbers: 82394463, 82425033, 92469108) and other grants, a team of land researchers from Shanghai Medical College, Fudan University, together with researchers Sun Lei and Professor Jiang Shibo, has made progress in the discovery of intermediate state targets for early fusion of coronavirus spike proteins and the design of antiviral drugs. The research results, titled "Early fusion intermediate of ACE2 using coronavirus spike acting as antiviral target", were published online on January 30, 2025 in the journal Cell. Paper link: https://doi.org/10.1016/j.cell.2025.01.012 .
The invasion of coronavirus into cells is mainly accomplished through membrane fusion mediated by the Spike (S) protein on the surface of the virus. The discovery of protein interactions and conformational features at various stages of S protein induced membrane fusion is of great significance for understanding the invasion mechanism of coronavirus and searching for new targets for anti coronavirus drug design to cope with current epidemics or future emerging coronavirus infectious diseases. The pre fusion conformation and receptor interaction of coronavirus S protein, as well as the post fusion conformation (i.e. hexahelix, 6HB), are relatively clear. However, the conformational characteristics of spike proteins during the transition from pre fusion state to post fusion state, especially in the early fusion intermediate state conformation, are still mainly in the speculative stage due to the difficulty in capturing their instantaneous conformational changes. Meanwhile, little is known about the antiviral drug targets present in this intermediate state.
The research team utilized molecular biology, virology, and other techniques to use SARS-CoV-2 as a model virus and discovered an early fusion intermediate conformation of its S protein that can simultaneously bind to ACE2 protein (i.e. RBD region of S1 subunit) and EK1 peptide (i.e. HR1 region of S2 subunit). Furthermore, a high-resolution early fusion intermediate conformation (E-FIC) of S protein of COVID-19 was obtained by using structural biology techniques. In E-FIC, the S2 subunit undergoes a conformational transition, where the HR1 triple helix and the IL770 complex containing S2 'in the middle ring region pop out, while the S1 subunit forms a cyclic structure and binds to the bottom of the S2 subunit. Based on the characteristics of the intermediate conformation mentioned above, researchers have identified potential antiviral targets and designed the protein based virus inactivator AL5E based on these targets. Through validation of in vivo and in vitro viral infection models, researchers have found that the AL5E protein has high efficiency and broad spectrum in rapidly reducing the infectivity of coronaviruses utilizing ACE2 receptors and inhibiting their infection (Figure).
This study revealed the details of the conformational changes experienced by the coronavirus S protein in the early stage of membrane fusion, and identified potential drug targets, providing ideas for further exploring the membrane fusion mechanism of coronaviruses and developing novel targeted broad-spectrum antiviral drugs or vaccines.