
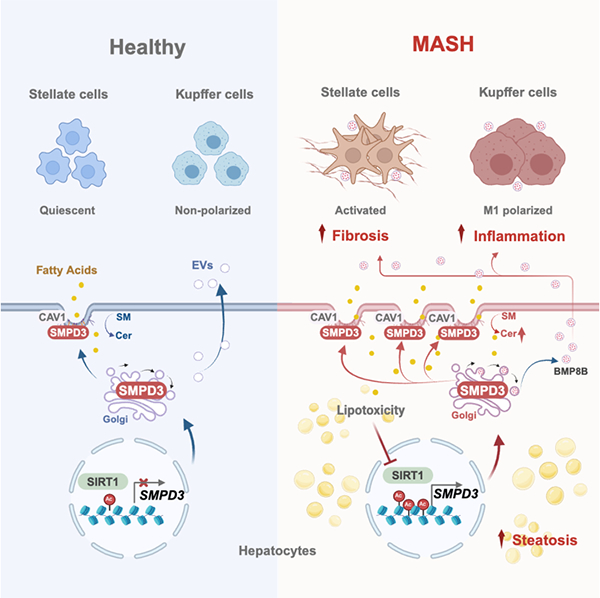
Schematic diagram of SMPD3 as a lipophilic receptor disrupting the balance
of cell membrane lipid metabolism and promoting the progression of MASH
Supported by the National Natural Science Foundation of China (Grant No.:
82222071, 22337003, 82322063), Xie Cen, a researcher from the Chinese Academy of
Sciences Shanghai Institute of Materia Medica, Xie Qing, a professor from Ruijin
Hospital affiliated to Shanghai Jiaotong University, Liu Hong, a researcher from
the Chinese Academy of Sciences Shanghai Institute of Materia Medica, and Liu
Yameng, an associate researcher, cooperated to make progress in the research of
original drug targets for steatohepatitis related to metabolic dysfunction. The
relevant results were published online on February 26, 2025 in the journal Cell
Metabolism under the title "Liver sphingomyelin phosphodiesterase 3 promotes
steatohepatitis by disrupting membrane sphingolipid metabolism". Paper link:
https://www.cell.com/cell-metabolism/fulltext/S1550-4131 (25)00016-6。
Metabolic dysfunction associated steatohepatitis (MASH) is the main cause
of cirrhosis and liver cancer, affecting approximately 25% of adults worldwide,
and there is currently a lack of effective intervention methods. MASH is
characterized by fatty degeneration, hepatocyte ballooning, inflammation, and
fibrosis as its main pathological features. Although inhibiting de novo lipid
synthesis can effectively reduce liver lipid accumulation, its effectiveness in
preventing the progression of MASH varies, and comprehensive inhibition of lipid
synthesis may affect membrane system stability and exacerbate liver damage.
Therefore, the core driving factor for the deterioration of MASH may not be the
total lipid burden, but rather the pathological cascade reactions triggered by
specific toxic lipids. Sphingolipids and their core metabolite ceramide are
considered key molecules involved in lipid toxicity, but the changes in the
sphingolipid metabolic network during MASH progression have not been
elucidated.
The research team analyzed the sphingolipid profile of MASH patients and
mouse models, and determined that sphingomyelin phosphodiesterase 3 (SMPD3) on
the cell membrane is a key driving factor in promoting the accumulation of
ceramides in MASH liver. Although SMPD3 expression is extremely low in healthy
liver, lipotoxicity induced DNA damage triggers pathological upregulation of
SMPD3 during MASH by inhibiting sirtuin 1 (SIRT1); SMPD3 enhances pit dependent
lipid uptake and extracellular vesicle secretion in steatotic liver cells by
disrupting the balance of sphingolipid metabolism in the small pit area of the
cell membrane, thereby exacerbating inflammation and fibrosis and promoting
disease progression. Therefore, the study proposes that SMPD3 is the central hub
and potential therapeutic target connecting key pathological features of MASH;
Through further small molecule compound dual target design and activity
screening, dual target compounds that simultaneously activate SIRT1 and inhibit
SMPD3 were discovered, demonstrating therapeutic potential superior to single
target drugs in various MASH animal models (Figure).
This study reveals that the imbalance of hepatic cell membrane lipid
metabolism is an important characteristic of MASH progression, and proposes
precise intervention of hepatic SMPD3 to restore membrane lipid metabolism or
help improve MASH. This research provides potential original drug targets and
lead compounds for the treatment of MASH.