
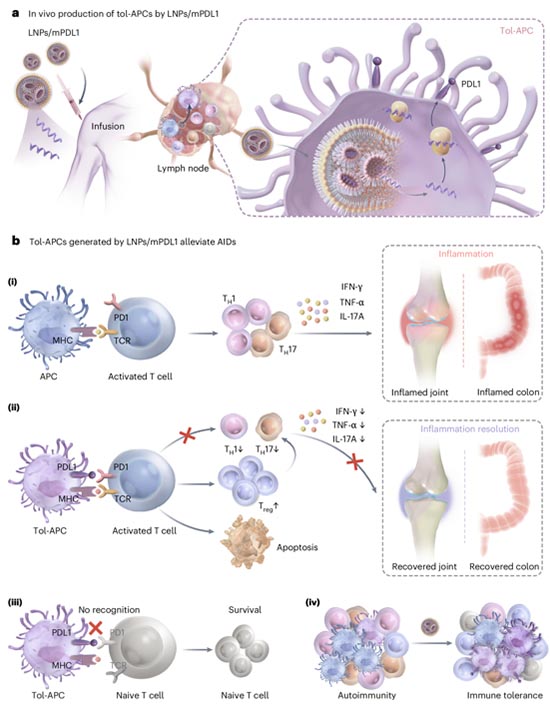
Figure Schematic diagram of low immunogenicity delivery of PDL1 mRNA in tol
APCs technology for in vivo generation of tolerant antigen presenting cells. (a)
The process of delivering PDL1 mRNA to tol APCs in vivo using low immunogenicity
lipid nanoparticles (LNPs); (b) The generated tol APCs selectively inhibit
activated T cells through the PDL1/PD1 pathway, effectively alleviating symptoms
of autoimmune diseases
With the support of National Natural Science Foundation of China projects
(approval numbers: 52025036, 82173390, 52495014), Professor Wang Yucai's team
from the Department of Life Sciences and Medicine at the University of Science
and Technology of China has made progress in the study of low immunogenicity
lipid nanoparticles (LNPs) delivering mRNA to generate tol APCs in vivo. The
related research results, titled "Generation of tolerogenic antigen presenting
cells in vivo through the delivery of mRNA encoding PDL1 within lipid
nanoparticles," were published online on March 28, 2025 in the journal Nature
Biomedical Engineering. The paper link is:
https://www.nature.com/articles/s41551-025-01373-0 .
In vivo cell engineering technology is considered an important direction
for future precision immunotherapy. This technology can achieve functional
reprogramming of target cells without the need for in vitro operations, and has
the advantages of scalability, standardization, and low cost. Based on this
emerging concept, this study developed a low immunogenicity LNP delivery
platform that achieves efficient and targeted delivery of PDL1 mRNA by
systematically regulating its N/P ratio and group allocation ratio, while
significantly reducing the stimulation of the immune system by nanoparticles
themselves. This system is capable of efficiently programming antigen-presenting
cells in vivo to obtain tol APC phenotype, effectively inducing pathogenic T
cell dysfunction and promoting regulatory T cell expansion. In animal models of
autoimmune diseases such as rheumatoid arthritis and ulcerative colitis, the tol
APCs generated by this strategy exhibit significant therapeutic effects,
accurately regulating immune balance and inhibiting disease progression. This
strategy breaks through the problems of complex operation, high cost, and
difficulty in popularizing individual customization in traditional in vitro
induction of tol APCs methods, significantly improving the clinical feasibility
of the technology.
Compared with conventional mRNA delivery systems widely used in vaccines,
the low immunogenicity LNP system developed in this study achieves a balance
between delivery efficiency and immune tolerance. Its characteristic is to
maintain efficient mRNA expression while avoiding APCs activation into immune
mature phenotype (such as upregulation of co stimulatory molecules such as
CD80/CD86/CD40), which is the key to achieving in vivo induction of tol APCs.
This study not only provides a new strategy for the treatment of autoimmune
diseases, but also provides technical support for the cutting-edge direction of
"in vivo immune cell engineering", with broad clinical translation
prospects.