
Response and function of SARS-CoV-2-specific T cells in bronchoalveolar lavage fluid
Under the support of the National Natural Science Foundation of China
(Grant No. 82495200, 82495203, 82025001, 82201933, 82201932), the team of
Professor Zhao Jincun, Professor Zhao Jingxian and Academician Zhong Nanshan
from the First Affiliated Hospital of Guangzhou Medical University/Guangzhou
National Laboratory made progress in the research on the characteristics and
functions of virus specific T cells in the lungs of COVID-19 patients. The
relevant research achievements are entitled "Robust mycosal SARS-CoV-2-specific
T cells effectively combat COVID-19 and establish multi-functional resident
memory in patient lungs", which was published online in Nature Immunology on
January 28, 2025. The link to the paper is:
https://www.nature.com/articles/s41590-024-02072-9 .
The COVID-19 epidemic has had a huge impact on global public health.
Although vaccination has played an important role in controlling the epidemic
situation, breakthrough infections still occur from time to time, suggesting
that we need to understand more about the human immune response, especially the
role of lung regional immune response in COVID-19 infection. Although the
research on novel coronavirus (SARS-CoV-2) continues to deepen, many key links
of the lung immune response, especially the characteristics and functions of
SARS-CoV-2 specific T cells in the lung, have not been thoroughly studied
before.
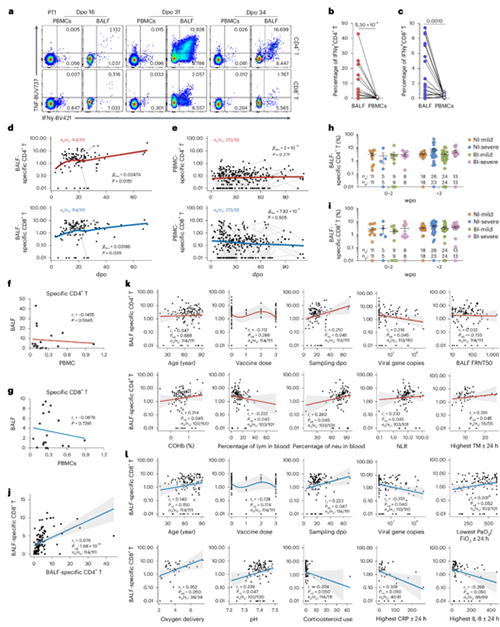
The research team included 159 COVID-19 patients and collected 122
bronchoalveolar lavage fluid (BALF) samples and 280 blood samples (including 27
pairs of bronchoalveolar lavage fluid and blood samples from 24 patients). A
comprehensive analysis of SARS-CoV-2-specific T cells in the lung airway and
periphery was conducted using techniques such as single-cell transcriptome
sequencing (scRNA seq), single-cell T cell receptor (TCR) sequencing, and
high-dimensional flow cytometry. The research team found that the number of
SARS-CoV-2-specific T cells in the bronchoalveolar lavage fluid of patients is
closely related to reduced viral load, reduced systemic inflammation, and
improved respiratory function, indicating that respiratory mucosal T cells play
an important role in controlling virus replication and reducing the severity of
COVID-19 (Figure). Moreover, compared with peripheral blood samples, the
specific T cells mentioned above exhibit stronger abilities to activate,
proliferate, and secrete multiple cytokines, and exhibit unique metabolic
characteristics mainly based on glycolysis, which can provide support for the
effector function of activated T cells. Further transcriptional analysis
revealed that interferon response, T cell activation, inflammation, tissue
migration, proliferation, and metabolism related pathways were significantly
upregulated in this type of virus specific T cells. After virus clearance, the
aforementioned T cells maintain a multifunctional tissue resident memory
phenotype, which can quickly respond to reinfection and provide long-term
protection against SARS-CoV-2. In addition, there was no significant difference
in the response of virus specific T cells in the lung region to SARS CoV-2
infection between the individuals who received intramuscular injection of
COVID-19 inactivated vaccine and those who did not receive the vaccine,
suggesting that intramuscular injection of vaccine may be difficult to establish
effective T cell immune memory in the airway, suggesting the necessity of
developing a mucosal vaccine that can induce local immunity in the respiratory
tract.
This study reveals the important role of SARS-CoV-2-specific T cells in
lung airway mucosa in COVID-19, providing a basis for developing more effective
mucosal vaccines to combat COVID-19 and other respiratory infections.