
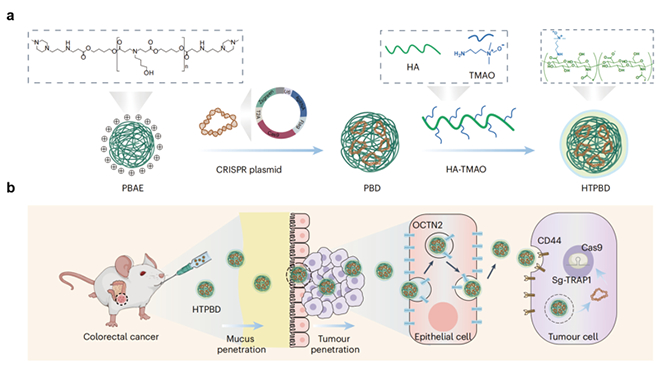
Figure (a) Schematic diagram of the preparation process of gene editing
oral delivery system; (b) Mechanism diagram of targeted gene editing for colon
cancer achieved by oral delivery system
With the support of the National Natural Science Foundation of China
projects (approval numbers: 52333004, 22135005), Professor Zhang Xianzheng's
team at Wuhan University has made progress in the research of gene editing oral
delivery systems. The related research results, titled "An orally administered
gene editing nanoparticle boosts chemo immunotherapy in colorectal cancer," were
published online on April 23, 2025 in the journal Nature Nanotechnology. The
paper link is: https://www.nature.com/articles/s41565-025-01904-5 .
The cyclopD (CypD) in mitochondria has been shown to be closely associated
with various inflammatory diseases. In colon cancer, the highly expressed tumor
necrosis factor receptor associated protein 1 (TRAP1) forms a stable complex
with CypD, hindering its translocation to the mitochondrial inner membrane and
inhibiting downstream inflammatory signaling. Therefore, targeted inhibition of
TRAP1 provides a potential strategy for enhancing chemotherapy sensitivity.
However, existing small molecule TRAP1 inhibitors generally have shortcomings
such as low mitochondrial targeting efficiency, poor selectivity, and poor
pharmacokinetic properties. In contrast, the CRISPR/Cas9 gene editing system has
the characteristic of achieving long-term regulation through short-term
administration, and the development of an oral CRISPR gene editing system with
better compliance in colorectal cancer patients has broad clinical application
prospects. However, oral gene editing systems still face multiple challenges:
firstly, the complex enzyme environment in the gastrointestinal tract can easily
lead to the degradation of nucleic acid drugs; Secondly, the physical barrier
formed by the tight junction of intestinal epithelium severely hinders the
absorption of large molecule drugs; In addition, existing delivery systems are
difficult to achieve tumor tissue-specific targeting and urgently require
breakthrough solutions.
In response to the above challenges, Professor Zhang Xianzheng's team has
confirmed that knocking out the TRAP1 gene with high expression in colon cancer
can effectively induce CypD membrane translocation, significantly enhancing
tumor necrosis and immune infiltration after chemotherapy. The research team
successfully constructed the oral gene editing delivery system HTPBD using
polysaccharide zwitterionic polymer (HA-TMAO) as the shell and poly (β -
aminoester) (PBAE) as the core. This system has high stability (resistant to
gastric acid and intestinal enzymes), excellent mucus diffusion ability, as well
as high tissue permeability and targeting. In addition, the system also has good
adaptability to freeze-dried formulations, which is conducive to long-term
storage and transportation. Due to the hydration of the outer zwitterionic TMAO,
HTPBD can rapidly penetrate the mucosal barrier and target enrichment in colon
cancer tissue through the endocytosis mediated by organic cation transporter 2
(OCTN2). The research results indicate that HTPBD has successfully reshaped the
immune microenvironment after chemotherapy in various colon cancer models,
demonstrating excellent tumor suppression effects and significantly enhancing
the therapeutic efficacy of chemoimmunotherapy. It has important clinical
translational potential and is particularly suitable for immune escape or
drug-resistant colon cancer patients.